PULMONARY EMBOLISM
|
We all know that shortness of breath and pleuritic chest pain may mean pulmonary embolism, however, we also know that not everything in medicine is clear-cut.
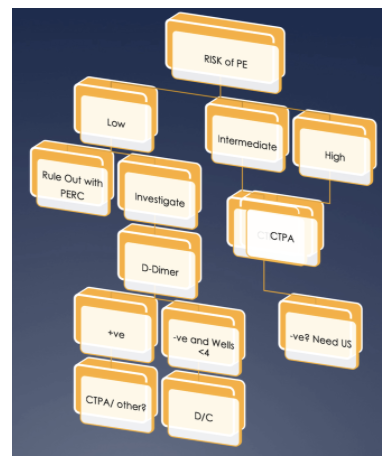
In terms of pulmonary embolism, it's all muddy waters. The following section will look at some of the symptoms and signs and determine if they are useful. we will also look at PE in pregnancy and look at the latest literature.. It's not about giving you the basics on PE, you have those. It's about giving you what's important. To the right is my brief overview of how I approach the patient with potential PE. The approach to subsegmental PE's is a massive area that needs more work. When you complete this section go to The ECG's of PE. |
Symptoms and Signs

Let's think about this statement a little.
Certainly some of the largest studies such as PIOPED found this. However, let's also remember that you had to have these to be included into the study.
Perhaps it's not as straighforward as we think.
In a major study looking at PE Stein P et al Chest 1991;100;598-603, looked at patients with suspected PE. PE was confirmed or excluded by angiogram.
Please answer the following question on Symptoms and then read on.
Certainly some of the largest studies such as PIOPED found this. However, let's also remember that you had to have these to be included into the study.
Perhaps it's not as straighforward as we think.
In a major study looking at PE Stein P et al Chest 1991;100;598-603, looked at patients with suspected PE. PE was confirmed or excluded by angiogram.
Please answer the following question on Symptoms and then read on.
DYSPNOEA, PLEURITIC CHEST PAIN, HEMOPTYSIS AND COUGH ARE CLEAR SIGNS OF PULMONARY EMBOLISM. TRUE/FALSE?
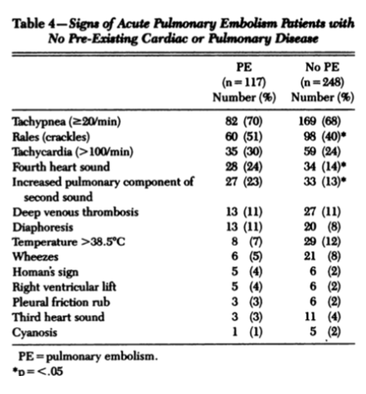
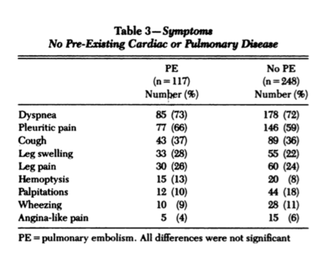
FALSE. What we've been taught is not necessarily true. If we look at this landmark study and the table below, we see that there is no statistical significance in terms of these symptoms in those with and without a PE.
SYMPTOMS
The most popular symptom is DYSPNOEA, however we see that the numbers of patients with this symptom that had PE and those that didn't was very similar.
To look at a great case that was thought to be a PE but fooled everyone, go to Case 3 in the SHOCKED module.
This is also true of pleuritic chest pain and cough. Although patients with haemoptysis seem to be greater in number in the PE group, this was not statistically significant.
The most popular symptom is DYSPNOEA, however we see that the numbers of patients with this symptom that had PE and those that didn't was very similar.
To look at a great case that was thought to be a PE but fooled everyone, go to Case 3 in the SHOCKED module.
This is also true of pleuritic chest pain and cough. Although patients with haemoptysis seem to be greater in number in the PE group, this was not statistically significant.
TACHYPNOEA IS THE MOST IMPORTANT SIGN IN DIAGNOSING PULMONARY EMBOLISM. TRUE OR FALSE?
ANSWER
Although tachypnoea is an important sign, it is not specific. It occurs for many reasons. Lung pathology such as infection, a pneumothorax or even a pericardial effusion can present with this. Let's look at the signs below.
|
SIGNS
We didn't have much luck with symptoms. How about signs? Let's look at what might be considered the most important of the signs:
|
THE IMPORTANT TAKE HOME POINT IS THAT IT IS A CONSTELLATION OF SYMPTOMS AND SIGNS THAT WILL POINT US TO THE DIAGNOSIS. NOT ONE SYMPTOM OR SIGN. THAT IS WHY GESTALT IS SUCH AN IMPORTANT WORD.
LAB INVESTIGATIONS
ABG
ABG'S: A PAO2 OF < 80 AND AN ABNORMAL A-A GRADIENT ARE DIAGNOSTIC OF A PE. TRUE OR FALSE?
ANSWER
Although gases may assist us in reaching a gestalt, they aren't that helpful. we know that:
- 18% of patients with a PE have a PaO2 >85mmHg
- We also know that the A-a gradient is normal in about 6% of patients with a PE.
D-dimer
WHO DO WE USE A D-DIMER ON?
aNSWER
The d-dimer is useful in the low probability patients with a Wells' score less than 4. A normal D-dimer with a low Wells score can rule out a pulmonary embolism.
WE SHOULDN'T DO A D-DIMER ON PATIENTS > 70 YEARS OLD AS WE KNOW IT WILL BE ELEVATED. TRUE OR FALSE?
ANSWER
Although this is something that used to be said. It no longer applies as we can now age adjust D-dimers. Read below.
AGE ADJUSTED D-DIMER
We use D-dimer in patients with a low probability of a pulmonary embolism, to rule out the condition and thus avoid imaging. The level of D-dimer rises with many conditions and also with age, thereby reducing it’s specificity for this condition. In a retrospective cohort study of D-dimer cut-offs, Ackerly et al(1) compared three proposals for D-dimer adjusted cut-offs in patients over 50 years of age.
We use D-dimer in patients with a low probability of a pulmonary embolism, to rule out the condition and thus avoid imaging. The level of D-dimer rises with many conditions and also with age, thereby reducing it’s specificity for this condition. In a retrospective cohort study of D-dimer cut-offs, Ackerly et al(1) compared three proposals for D-dimer adjusted cut-offs in patients over 50 years of age.
Proposal 1
age (years) x 10 (2)
Proposal 2
age (years) x 16 (3)
Proposal 3 (4)
Decade specific cut-offs
-< 60yo 500 mcg/L
-61-70 600 mcg/L
-71-80 700 mcg/L
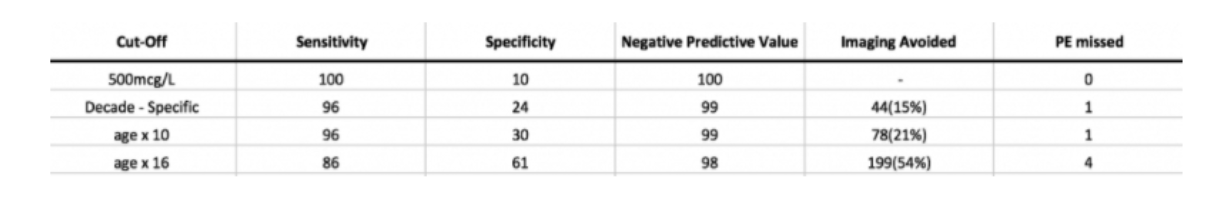
Results are shown:
It is important to know that the decade specific and age x 10 PE’s that were missed were sub-segmental. half of the age x 16 were sub-segmental. It comes back to the question of what is an acceptable cut-off? How many are you prepared to miss?
I agree with the authors that the age x 10 is easy to remember and apply.
age (years) x 10 (2)
Proposal 2
age (years) x 16 (3)
Proposal 3 (4)
Decade specific cut-offs
-< 60yo 500 mcg/L
-61-70 600 mcg/L
-71-80 700 mcg/L
Results are shown:
It is important to know that the decade specific and age x 10 PE’s that were missed were sub-segmental. half of the age x 16 were sub-segmental. It comes back to the question of what is an acceptable cut-off? How many are you prepared to miss?
I agree with the authors that the age x 10 is easy to remember and apply.
Thrombolysis in PE
CASE
A 78 yo male presents with shortness of breath. He has a past medical history of hypertension. His vitals are HR 114, BP 128/80,RR 28, Sats 91% on room air. He has no chest pain and is sitting up feeling very comfortable with supplementary oxygen.
Should this patient be offered thrombolysis? Would you give thrombolysis?
Massive PE
Traditionally this has been defined by the angiographic burden, however a more appropriate definition may be related to what we see clinically. I prefer the American Heart Association(AHA)(1) definition:
“Acute PE with sustained hypotension (systolic blood pressure <90 mm Hg for at least 15 minutes or requiring inotropic support, not due to a cause other than PE, such as arrhythmia, hypovolaemia, sepsis, or left ventricular [LV] dysfunction), pulseless, or persistent profound bradycardia (heart rate <40 bpm with signs or symptoms of shock).”
Submassive PE
Submassive PE is defined by the AHA(1) as:
“Acute PE without systemic hypotension (systolic blood pressure ≥90 mm Hg) but with either RV dysfunction or myocardial necrosis.”
- RV dysfunction means the presence of at least RV dilation or RV systolic dysfunction on echocardiography, RV dilation on CT, Elevation of BNP (>90 pg/mL), Elevation of N-terminal pro-BNP (>500 pg/mL); or
- Electrocardiographic changes (new complete or incomplete right bundle-branch block, anteroseptal ST elevation or depression, or anteroseptal T-wave inversion)
- Myocardial necrosis is defined as either of Elevation of troponin I (>0.4 ng/mL) or Elevation of troponin T (>0.1 ng/mL)
Who Gets Thrombolysis?
It’s generally accepted that massive pulmonary embolism gets thrombolysed, although there may be some contraindications. What do we do with submassive PE’s? Are some of them deserving of lysis? Until now it has been accepted that those patients with haemodynamic stability and RV dysfunction and myocardial necrosis should be candidates for thrombolysis.
There is no doubt that the use of thrombolytic significantly reduces clot size. This may have the direct result of decreasing pulmonary pressures, which may themselves lead to future complications. However, there is no significant effect on patient mortality(2). In fact, in normotensive patients thrombolytics are associated with increased mortality(3), with the primary complication being intracranial haemorrhage, in up to 6.4% of patients(4).
There are several trials, however two are considered major trials, the MOPETT and the PEITHO trials. Even with these, the question still hasn’t really been answered. The two trials use a different type of lytic agent and different endpoints.
The MOPETT Trial(5)
This trial wanted to address the concerns of full dose thrombolysis and potential intracranial bleeding, by proposing a ‘safe dose’ of 0.5mg/kg to a maximum of 50mg (50%) of the regular dose of tPA. It was given as 10mg over one minute and the rest within a 2 hour period.
This was a single centre, small numbered study, that really didn’t find any statistically significant results in reduction of pulmonary hypertension. It is questionable as to whether the patients chosen, were all appropriate, as not all met the criteria of submissive PE. What did come out of it was that there were no cases of intra-cerebral bleed with half dose tPA. This is a very important finding.
The PEITHO Trial (6)
This was a trial of 1006 patients treated with Tenecteplase. Although the results didn’t for the most reach statistical significance, the trend towards haemodynamic compromise was much higher in the placebo group than the Tenecteplase group. The rate of major bleeding was 6.3% in the thrombolytic group and the rate of intra-cranial bleeding was 2% with tenecteplace versus 0.2% with placebo. Those at greater risk appeared to be those patients older than 75 years.
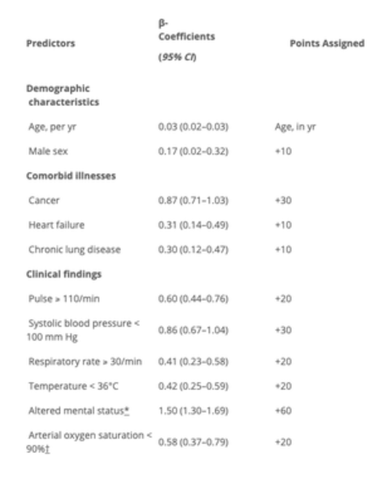
Pulmonary Embolism Severity IndexThe Pulmonary Embolism Severity Index is a validated score that provides independent predictors of 30 day mortality and can be used to determine those patients at higher risk(6). This may help us in determining those patients that will have a worst outcome if not treated.
The scoring system is shown below:
How does this apply to our patient?
The patient in the case above, would fall into the HIGH RISK group in terms of poor outcome. He would score a 78(age) + 10(male) + 20(HR) =108.
Thrombolysis should be considered in him, however I would consider half dose if tPA were being given and would get advice on Tenecteplase.
Overall Approach
We need to consider the risk of intra-cerebral bleeding. Given this, many would thrombolyse only massive PE’s, although in those who are haemodynamically stable we would look for RV strain or cardiac necrosis,
The alternative is to calculate a PE Index and consult with a pulmonary hypertension expert.
Special Cases
Recent Surgery
The risk of bleeding if patient have had recent surgery is significant in the first 2 weeks after surgery. That risk falls two weeks following surgery.
Is all surgery the same? No, brain or spinal surgery does not carry the same risks.
Recent Stroke
There is some concern with giving thrombolysis within 3-6 months of ischaemic stroke, in an increased rate of intracranial haemorrhage(7), although the evidence does not appear to be consistent. Specialist advise should be sought.
Space Occupying Lesions
There appears to be an increased risk of haemorrhage in all except meningiomas(8). Expert advice should be sought.
The patient in the case above, would fall into the HIGH RISK group in terms of poor outcome. He would score a 78(age) + 10(male) + 20(HR) =108.
Thrombolysis should be considered in him, however I would consider half dose if tPA were being given and would get advice on Tenecteplase.
Overall Approach
We need to consider the risk of intra-cerebral bleeding. Given this, many would thrombolyse only massive PE’s, although in those who are haemodynamically stable we would look for RV strain or cardiac necrosis,
The alternative is to calculate a PE Index and consult with a pulmonary hypertension expert.
Special Cases
Recent Surgery
The risk of bleeding if patient have had recent surgery is significant in the first 2 weeks after surgery. That risk falls two weeks following surgery.
Is all surgery the same? No, brain or spinal surgery does not carry the same risks.
Recent Stroke
There is some concern with giving thrombolysis within 3-6 months of ischaemic stroke, in an increased rate of intracranial haemorrhage(7), although the evidence does not appear to be consistent. Specialist advise should be sought.
Space Occupying Lesions
There appears to be an increased risk of haemorrhage in all except meningiomas(8). Expert advice should be sought.
What about PE in Pregnancy?
The confusion in PE is related to:
Here are some thoughts.
- Acceptable radiation risk. There are two distinct risks, that to the mother and that to the fetus and
- D-Dimer Use.
Here are some thoughts.
THE RISK OF PE IN PREGNANCY IS SIMILAR TO THAT OF THE NON-PREGNANT PATIENT. TRUE OR FALSE?
FALSE
FALSE
The risk is higher in this patient group; five times the risk in a non-pregnant patient. This automatically raises the risk to, or above 15%.
The risk is higher in this patient group; five times the risk in a non-pregnant patient. This automatically raises the risk to, or above 15%.
DIAGNOSING PE IN PREGNANCY IS DIFFICULT AS THE SYMPTOMS OF PREGNANCY AND PE ARE SIMILAR.
TRUE OR FALSE?
TRUE OR FALSE?
ANSWER
TRUE
The signs and symptoms that accompany pregnancy, such as;
– shortness of breath
– tachycardia
– leg oedema
can also be present in patients with pulmonary embolism.
Gherman et al Obstet Gynecol 1999; 94; 730-734 found that in pregnant patients with confirmed PE
– 62% had dyspnoea
– 50% had pleuritic chest pain
– 24% had cough
– 18% had sweating
but this was a very small study (n=38)
The signs and symptoms that accompany pregnancy, such as;
– shortness of breath
– tachycardia
– leg oedema
can also be present in patients with pulmonary embolism.
Gherman et al Obstet Gynecol 1999; 94; 730-734 found that in pregnant patients with confirmed PE
– 62% had dyspnoea
– 50% had pleuritic chest pain
– 24% had cough
– 18% had sweating
but this was a very small study (n=38)
THE WELLS SCORE CAN HELP US RULE OUT PE IN PREGNANT PATIENTS WITH A LOW PRE-TEST PROBABILITY.
TRUE OR FALSE?
TRUE OR FALSE?
FALSE
FALSE
The Wells score cannot be used as pregnant patients were excluded from the validation study.(Ann Int Med 2001 135:98-107).
The Wells score cannot be used as pregnant patients were excluded from the validation study.(Ann Int Med 2001 135:98-107).
CAN WE USE D-DIMER IN PREGNANCY?
PERFORMING A CHEST XRAY ON A PREGNANT PATIENT IS LOW RADIATION AND IF WE FIND A CLASSICAL WESTERMARK SIGN WE CAN STOP. TRUE OR FALSE?
ANSWER
FALSE- Please read below.
READ ON V/Q, MRA, ULTRASOUND AND CHEST X-RAY
V/Q, CTPA, MRA, Ultrasound and chest X-ray
Currently MR pulmonary angiograms are not considered for use, as to my knowledge, there have not not been any studies done in the pregnant patient. Secondly, although we know, like iodinated contrast agents, gadolinium also crosses the placenta, we are really unsure of its effect on the fetus and even though there have been no adverse fetal effects reported from Gadolinium, the studies are few. In terms of sensitivity and specificity, the MR angiogram approaches that, but may not be as good as, CTPA(AJR 2006 Jul; 187(1):109-114).
What about the ultrasound?
The ultrasound can and should be the first investigation performed. It is simple, non-invasive and does not expose the patient to any ionizing radiation. Approximately 70% of patients with PE have proximal deep vein thrombosis. We do not actually know the prevalence of DVT in pregnant patients presenting with a suspected PE.
We do know that the prevalence of DVT in those patients without symptoms is low. However if you, suspect a PE and find a DVT on ultrasound you’re done.
Is there a place for chest X-Ray?
My view is that there is no major role for this investigation in the diagnosis of PE. There are no real sensitive or specific findings for a PE on chest x-ray. Even if we find a Westermark sign we will not make the diagnosis based on this.
The chest x-ray may be of use for those undergoing PE, to allow correlation of findings.
What about the V/Q scan?
The major concerns with V/Q scans have been that, in the general population, up to 70% of scans are indeterminate.
The pregnant patient however, does not fall into the normal population group. In the pregnant patient less than 5% of scans are high probability and 75% of scans are normal.(Arch Int Med 2002; 162:1170-5). This is probably as a result of this population group being younger, with less co-morbidities. This means that only about 20% of V/Q scans are indeterminate.
The radiation dose of the V/Q scan varies but can be further reduced, by using a half dose perfusion scan. Detection of a defect means no ventilation scan is required.
In the mother, the malignancies we are concerned about, are related to lung and breast. The maternal whole body radiation dose of a V/Q scan is 1-2.5 mSv. The radiation dose to breast tissue is 0.28-0.5mSv(Europ Radiol 2003; 13:1515-1521)
The lifetime risk of breast cancer from 20mGy is
– 1/1200 women at age 20
– 1/2000 women at age 30
– 1/3500 women at age 40
The lifetime risk of breast cancer is 1/8.
Perfusion only V/Q scans significant;y reduce exposure. Scarbrook et al Eur Radiol 2007:17:2554-2560 reported on the use of perfusion-only scans and found a 100% negative predictive value of PE for scans other than high probability.
What about CTPA?
With the increase availability of CT scanners, it is very simple to perform a CTPA.
It has a high sensitivity and specificity. A negative predictive value for PE following a normal scan is >99%(JAMA 2005; 293:2012-7)
In a retrospective study comparing CTPA and V/Q by Shahir et al Am J Roentg 2010; 195:W214-220 found a negative predictive value of 99% and 100% respectively.
The fetal radiation dose increases with each trimester, from 3.3mGy to 130mGy. In terms of fetal radiation exposure, there is a trend that favours CTPA for the 1st and second trimesters of pregnancy(BMJ 2005; 331:350)
The concern with CTPA is the maternal radiation dose.
The literature on maternal risk of cancer can be confusing.
There is evidence that greater than 1000mGy is associated with a 40% increase in the risk of breast cancer. We are told that there is no increased risk at below 200mGy(Lancet 2010; 375:500-512). The CTPA radiation dose doesn’t come anywhere near this.
We do however know that there is an increased risk of breast cancer in over 35 year olds with a 10mGy dose to the breast. We also know that the overall, lifetime risk of breast cancer is 0.7% (JAMA 2007; 298:317-23) Further, the predictive models that we have show a higher lifetime risk of radiation induced cancer from a CTPA rather than a V/Q scan.
Currently MR pulmonary angiograms are not considered for use, as to my knowledge, there have not not been any studies done in the pregnant patient. Secondly, although we know, like iodinated contrast agents, gadolinium also crosses the placenta, we are really unsure of its effect on the fetus and even though there have been no adverse fetal effects reported from Gadolinium, the studies are few. In terms of sensitivity and specificity, the MR angiogram approaches that, but may not be as good as, CTPA(AJR 2006 Jul; 187(1):109-114).
What about the ultrasound?
The ultrasound can and should be the first investigation performed. It is simple, non-invasive and does not expose the patient to any ionizing radiation. Approximately 70% of patients with PE have proximal deep vein thrombosis. We do not actually know the prevalence of DVT in pregnant patients presenting with a suspected PE.
We do know that the prevalence of DVT in those patients without symptoms is low. However if you, suspect a PE and find a DVT on ultrasound you’re done.
Is there a place for chest X-Ray?
My view is that there is no major role for this investigation in the diagnosis of PE. There are no real sensitive or specific findings for a PE on chest x-ray. Even if we find a Westermark sign we will not make the diagnosis based on this.
The chest x-ray may be of use for those undergoing PE, to allow correlation of findings.
What about the V/Q scan?
The major concerns with V/Q scans have been that, in the general population, up to 70% of scans are indeterminate.
The pregnant patient however, does not fall into the normal population group. In the pregnant patient less than 5% of scans are high probability and 75% of scans are normal.(Arch Int Med 2002; 162:1170-5). This is probably as a result of this population group being younger, with less co-morbidities. This means that only about 20% of V/Q scans are indeterminate.
The radiation dose of the V/Q scan varies but can be further reduced, by using a half dose perfusion scan. Detection of a defect means no ventilation scan is required.
In the mother, the malignancies we are concerned about, are related to lung and breast. The maternal whole body radiation dose of a V/Q scan is 1-2.5 mSv. The radiation dose to breast tissue is 0.28-0.5mSv(Europ Radiol 2003; 13:1515-1521)
The lifetime risk of breast cancer from 20mGy is
– 1/1200 women at age 20
– 1/2000 women at age 30
– 1/3500 women at age 40
The lifetime risk of breast cancer is 1/8.
Perfusion only V/Q scans significant;y reduce exposure. Scarbrook et al Eur Radiol 2007:17:2554-2560 reported on the use of perfusion-only scans and found a 100% negative predictive value of PE for scans other than high probability.
What about CTPA?
With the increase availability of CT scanners, it is very simple to perform a CTPA.
It has a high sensitivity and specificity. A negative predictive value for PE following a normal scan is >99%(JAMA 2005; 293:2012-7)
In a retrospective study comparing CTPA and V/Q by Shahir et al Am J Roentg 2010; 195:W214-220 found a negative predictive value of 99% and 100% respectively.
The fetal radiation dose increases with each trimester, from 3.3mGy to 130mGy. In terms of fetal radiation exposure, there is a trend that favours CTPA for the 1st and second trimesters of pregnancy(BMJ 2005; 331:350)
The concern with CTPA is the maternal radiation dose.
The literature on maternal risk of cancer can be confusing.
There is evidence that greater than 1000mGy is associated with a 40% increase in the risk of breast cancer. We are told that there is no increased risk at below 200mGy(Lancet 2010; 375:500-512). The CTPA radiation dose doesn’t come anywhere near this.
We do however know that there is an increased risk of breast cancer in over 35 year olds with a 10mGy dose to the breast. We also know that the overall, lifetime risk of breast cancer is 0.7% (JAMA 2007; 298:317-23) Further, the predictive models that we have show a higher lifetime risk of radiation induced cancer from a CTPA rather than a V/Q scan.
Watch this video of lecture given by Dr Adam Michael on PE in pregnancy
Sub-segmental Pulmonary Embolism
ALL SUB-SEGMENTAL PULMONARY EMBOLISM NEEDS TREATMENT. TRUE OR FALSE?
ANSWER
This is such a difficult area. it's a new area. The significance of sub-segmental PE's has been questioned.
We hear the cry, "Isn't the role of the lungs to clear subsegmental PE's" and "The only reason we find them is because of the increased sensitivity of our CT scanners?"
The reality is that we don't know what the answer is. There are some patients at higher risk.
We hear the cry, "Isn't the role of the lungs to clear subsegmental PE's" and "The only reason we find them is because of the increased sensitivity of our CT scanners?"
The reality is that we don't know what the answer is. There are some patients at higher risk.
The American College of Chest Physicians has just produced a treatment guideline for venous thromboembolism(VTE) and Pulmonary Embolism(PE). The primary push in this guideline is for the use of non-vitamin K oral anticoagulants over warfarin.
In subsegmental PE and no Proximal DVT, in patients with a low risk of VTE: clinical surveillance over anticoagulation is recommended.
Clive Kearon, Elie A. Akl, Joseph Ornelas, Allen Blaivas, David Jimenez, Henri Bounameaux, Menno Huisman, Christopher S. King, Timothy Morris, Namita Sood, Scott M. Stevens, Janine R.E. Vintch, Philip Wells, Scott C. Woller, COL Lisa Moores. Antithrombotic Therapy for VTE Disease. Chest, 2016; DOI: 10.1016/j.chest.2015.11.026
Watch the lecture by Dr Gerben Keijzers on sub-segmental PE's
In subsegmental PE and no Proximal DVT, in patients with a low risk of VTE: clinical surveillance over anticoagulation is recommended.
Clive Kearon, Elie A. Akl, Joseph Ornelas, Allen Blaivas, David Jimenez, Henri Bounameaux, Menno Huisman, Christopher S. King, Timothy Morris, Namita Sood, Scott M. Stevens, Janine R.E. Vintch, Philip Wells, Scott C. Woller, COL Lisa Moores. Antithrombotic Therapy for VTE Disease. Chest, 2016; DOI: 10.1016/j.chest.2015.11.026
Watch the lecture by Dr Gerben Keijzers on sub-segmental PE's
Scoring Systems
The idea of scoring systems is to have them apply to a low clinical probability case to rule out. A few comments:
|
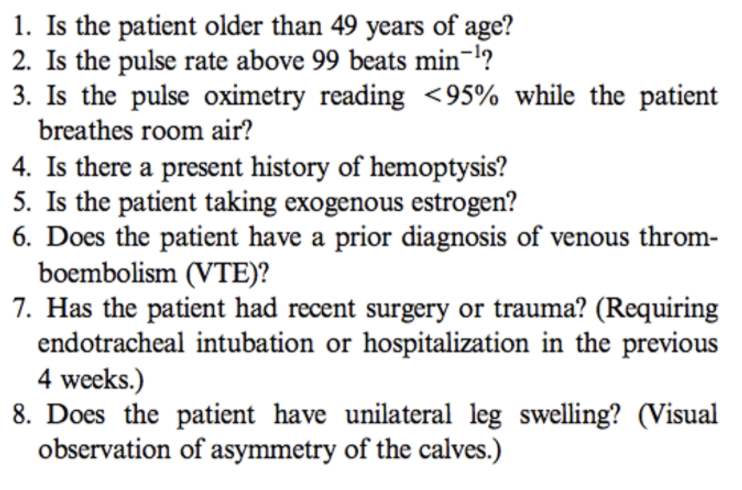
The Pulmonary Embolism Rule-out Criteria (PERC Rule
|
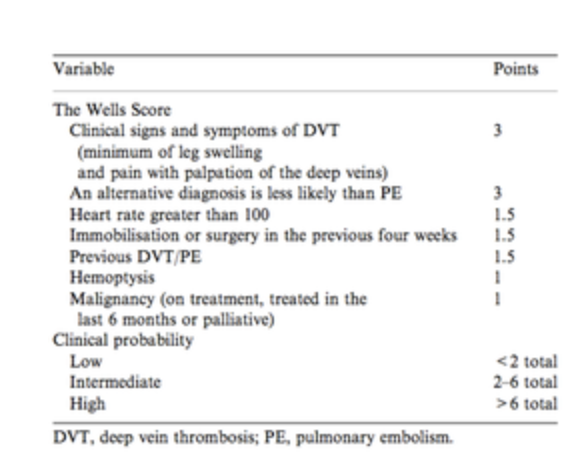
The Wells Score
|
|
—97.5% Sensitivity 22% Specificity
—It is for low risk patients, that can probably send them home. —The potential for harm with further investigations is higher than the miss rate —GOOD RULE TO RULE OUT WITH —If negative don’t need a d-dimer BEWARE The patient MUST NOT be tachycardic at any time |
A wells score of 4 or less and a negative QUANTITATIVE D-dimer in a patient where your gestalt, is that they are low risk, rules them out and no further imaging is necessary.
|
REFERENCES
1 Ackerly I et al. Which age adjusted D-dimer cut-off performs best? EMA Vol 29, Issue 5, October 2017
2 Flores J et al. Clinical usefulness and safety of an age-adjusted D-Dimer cut-off levels to exclude pulmonary embolism: a retrospective analysis. Intern Emerg Med 2016;11:69-75
3 Verma N et al. Age-adjusted D-dimer cut-offs to diagnose thromboembolic events: validation in an emergency department. Med Klin Intensivmed Norfmed. 2014;109:121-128
4 Gupta A et al. Assessing a 2 D-dimer age-adjustment strategies to optimise computed tomographic use in ED evaluation of pulmonary embolism. Am J. Emerg. Med. 2014;32:1499-1502.
2 Flores J et al. Clinical usefulness and safety of an age-adjusted D-Dimer cut-off levels to exclude pulmonary embolism: a retrospective analysis. Intern Emerg Med 2016;11:69-75
3 Verma N et al. Age-adjusted D-dimer cut-offs to diagnose thromboembolic events: validation in an emergency department. Med Klin Intensivmed Norfmed. 2014;109:121-128
4 Gupta A et al. Assessing a 2 D-dimer age-adjustment strategies to optimise computed tomographic use in ED evaluation of pulmonary embolism. Am J. Emerg. Med. 2014;32:1499-1502.